Abstract

[Background] Allogeneic hematopoietic stem cell transplantation (HSCT) is a potent treatment to cure in patients with aplastic anemia (AA). However, an optimal pre-transplant conditioning remains unclear. The combination of high-dose cyclophosphamide (CY) and anti-thymocyte globulin (ATG) has been used as an effective conditioning, but cardiotoxity due to high-dose CY has been a major concern especially in patients with iron overload by excessive transfusion. In addition, the appropriate dose and timing of ATG use is another unsolved topic in HSCT for AA. Therefore, we performed a prospective study to assess the safety and efficacy of a conditioning regimen using fludarabine (Flu), reduced-dose CY, and low-dose thymoglobulin in HSCT for AA.
[Methods] Patients with severe AA, aged between 16 and 65 years, who have an HLA-matched or 1-locus mismatched, related or unrelated donor were prospectively included. A conditioning regimen consisted of Flu 30mg/m2 for 4 days, CY 25mg/kg for 4 days, and thymoglobulin 1.25mg/kg for 2 days (days -4, and -3). In patients who underwent transplantation from unrelated and/or HLA-mismatched donor, 2 Gy of total body irradiation was added. Cyclosporine for an HLA-matched related donor or tacrolimus for the other donors, together with short-term methotrexate were used as graft-versus-host disease (GVHD) prophylaxis. Granulocyte-colony stimulating factor was used from 1 day after transplantation. Primary outcome measure was an overall survival at 1 year after HSCT. This study was approved by the Institutional Review Board of all the participating institutions.
[Results] Twenty-eight patients were enrolled between 2011 and 2017, and their median age was 36 years (range: 18 - 61y). Sixteen patients were male. A median time from diagnosis to transplantation was 1858 days in patients who had previously received immunosuppressive therapy (IST) using ATG (n = 16) and 121 days in those who had not received IST (n = 12). Sixteen patients received graft form related donors including 1 HLA-mismatched donor, and 12 patients did from unrelated donors including 3 mismatched donors. Stem cell source was bone marrow in 27 out of 28 patients.
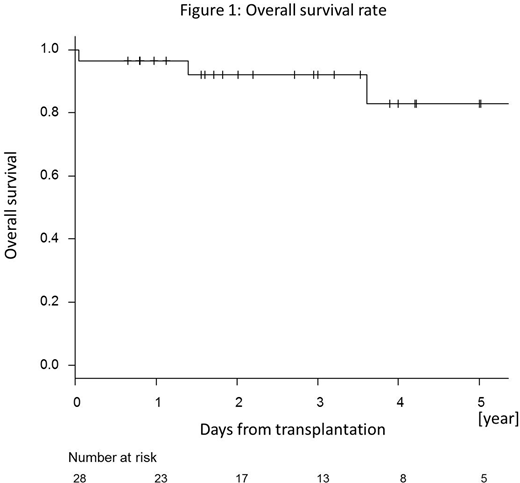
All patients but one, who died early due to infection, achieved neutrophil engraftment at a median of 19 days after HSCT. Mixed chimerism (MC) was observed in 6 patients at day 30, and 3 out of those 6 patients achieved complete donor chimerism by day 90. On the other hand, MC was newly observed in additional 2 patients at day 90. Only one patient experienced secondary engraftment failure, and which developed with complete donor-type chimerism at day 99. No patients developed grade 2-4 acute GVHD. However, the cumulative incidence of chronic GVHD was 39.9 % at 1 year, and one third of them had extensive type. With a median follow-up period of 1727 days for survivors, overall survival rates (OS) were 96.4 % at 1 year and 82.8 % at 5 years (Figure 1).
Cytomegalovirus (CMV) antigenemia was detected in 17 patients, but no patients developed CMV disease. A high Epstein-Barr virus (EBV)-DNA load (more than 1×104 copies/mL) was detected in 2 patients at day 60 and 1 patient at day 90. Neither developed EBV-lymphoproliferative disorder (LPD) within a year. However, another patient, in whom high EBV-DNA load was not detected within 90days after HSCT, developed EBV-LPD and died at three and a half years after HSCT.
[Conclusion] HSCT for AA using Flu, reduced-dose CY, and low-dose thymoglobulin as a conditioning regimen was safe and effective. Relatively high incidences of MC and chronic GVHD need further improvement. (This study is registered with www.umin.ac.jp as UMIN000006071.)
Kako:Takeda Pharmaceutical Company Limited.: Honoraria; Takeda Pharmaceutical Company Limited.: Honoraria; Celgene K.K.: Honoraria; Bristol-Myers Squibb: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Ono Pharmaceutical Co., Ltd.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria. Kanda:Tanabe-Mitsubishi: Research Funding; Shionogi: Consultancy, Honoraria, Research Funding; Taisho-Toyama: Research Funding; Pfizer: Research Funding; Taiho: Research Funding; MSD: Research Funding; Sanofi: Research Funding; Novartis: Research Funding; Eisai: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Otsuka: Research Funding; Asahi-Kasei: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; CSL Behring: Research Funding; Ono: Consultancy, Honoraria, Research Funding; Nippon-Shinyaku: Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Kyowa-Hakko Kirin: Consultancy, Honoraria, Research Funding; Dainippon-Sumitomo: Consultancy, Honoraria, Research Funding; Chugai: Consultancy, Honoraria, Research Funding; Takara-bio: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Mochida: Consultancy, Honoraria; Alexion: Consultancy, Honoraria. Usuki:Ono Pharmaceutical: Speakers Bureau; GlaxoSmithKline K.K.: Research Funding; Janssen Pharmaceutical K.K: Research Funding; Sanofi K.K.: Research Funding; Shire Japan: Research Funding; SymBio Pharmaceuticals Limited.: Research Funding; Celgene Corporation: Research Funding, Speakers Bureau; Daiichi Sankyo: Research Funding; Takeda Pharmaceutical: Speakers Bureau; Boehringer-Ingelheim Japan: Research Funding; Pfizer Japan: Research Funding, Speakers Bureau; Sumitomo Dainippon Pharma: Research Funding, Speakers Bureau; Novartis: Speakers Bureau; Chugai Pharmaceutical: Speakers Bureau; Kyowa Hakko Kirin Co., Ltd.: Research Funding; Otsuka Pharmaceutical Co., Ltd.: Research Funding; Astellas Pharma Inc.: Research Funding; Nippon Shinyaku: Speakers Bureau; Mochida Pharmaceutical: Speakers Bureau; MSD K.K.: Speakers Bureau. Mori:Astella Pharma: Honoraria; Shire Japan: Honoraria; Janssen: Honoraria; SHIONOGI: Honoraria; Taisho Toyama Pharmaceutical Co: Honoraria; Novartis Pharma: Honoraria; Celgene: Honoraria; Japan Blood Products Organization: Honoraria; Pfizer: Honoraria; CHUGAI: Honoraria; MSD: Honoraria; Eisai: Honoraria; Novartis Pharma: Research Funding; Asahi Kasei: Research Funding; MSD: Research Funding; Kyowa Hakko Kirin: Honoraria; Ono: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal